

![]()
The search for clean, renewable energy sources is one of the critical issues of our day; the matter of energy storage is equally important as energy generation. Along with the idea of using hydrogen as the fuel for fuel cell cars comes the question of how best to store hydrogen. For mobile energy storage purposes, containment must be lightweight, compact, and safe. Complex metal hydrides offer one potential solution to this problem. Complex metal hydrides store hydrogen by forming an ionic or covalent compound between the metals and hydrogen. These systems boast both a high volumetric density and mass density of potential hydrogen storage. However, thermodynamics and kinetics of hydrogen uptake and release remain significant challenges to finding a viable material that satisfies the stated requirements. It has been shown that the addition of small amounts of certain metals (e.g., titanium, scandium, iron and nickel) modify the thermodynamics of the reactions, the reason behind this enhancement is not currently understood. To gain a better understanding of how catalyst species impact the uptake and release of hydrogen, I am using STM to probe structural and electronic changes that occur on exposing a high purity Al surface with and without Ti to molecular hydrogen. The images below show some preliminary results of the formation of vacancies and alane, AlH3, on a Ti-doped Al surface. This reaction occurs in the presence of Ti only.
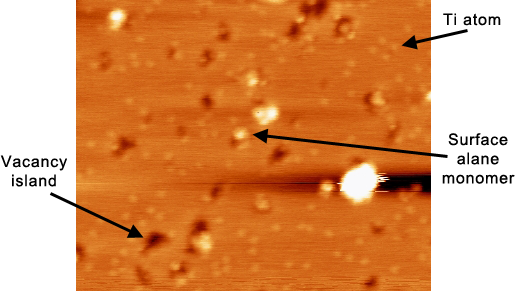
Figure 1. Al island surface showing Ti atoms (the all small, widespread light dots), vacancy islands (large dark spots), and surface alanes (the very bright spots) of varying sizes. The vacancies and alanes formed upon hydrogenation of the surface with H2.
Scan parameters: scan size = 24x19.7 nm2, V = -1.441 V, I = 0.264 nA.
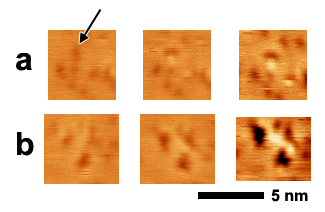
Figure 2. In (a) a vacancy island near the indicated Ti atom, and shortly thereafter an alane monomer forms at the edge of the vacancy. In (b) a group of vacancy islands grow, followed by the formation of a string of three alane monomers between the vacancies. Each series is pulled from a set of sequential scans taken at a scan rate of 85 nm/s.
PhD in Materials Science and Engineering
BS in Materials Science and Engineering, UIUC (2008)
Study of Hydrogen Storage Materials using STM
Electron Tomography
Electron Tomography